About us
Background and Goals of the CRC/TRR 333 BATenergy
Energy homeostasis and adipose tissues
Energy homeostasis is a central physiological process and energy imbalances can have severe health consequences. Approximately two billion people world-wide are affected by overweight and obesity, which are caused by a surplus in energy intake. Obesity substantially increases the risk for some of the most common chronic and fatal illnesses including heart disease, type 2 diabetes and cancer. There is an urgent need for coordinated programs to enhance scientific progress and to develop novel preventive and therapeutic approaches.
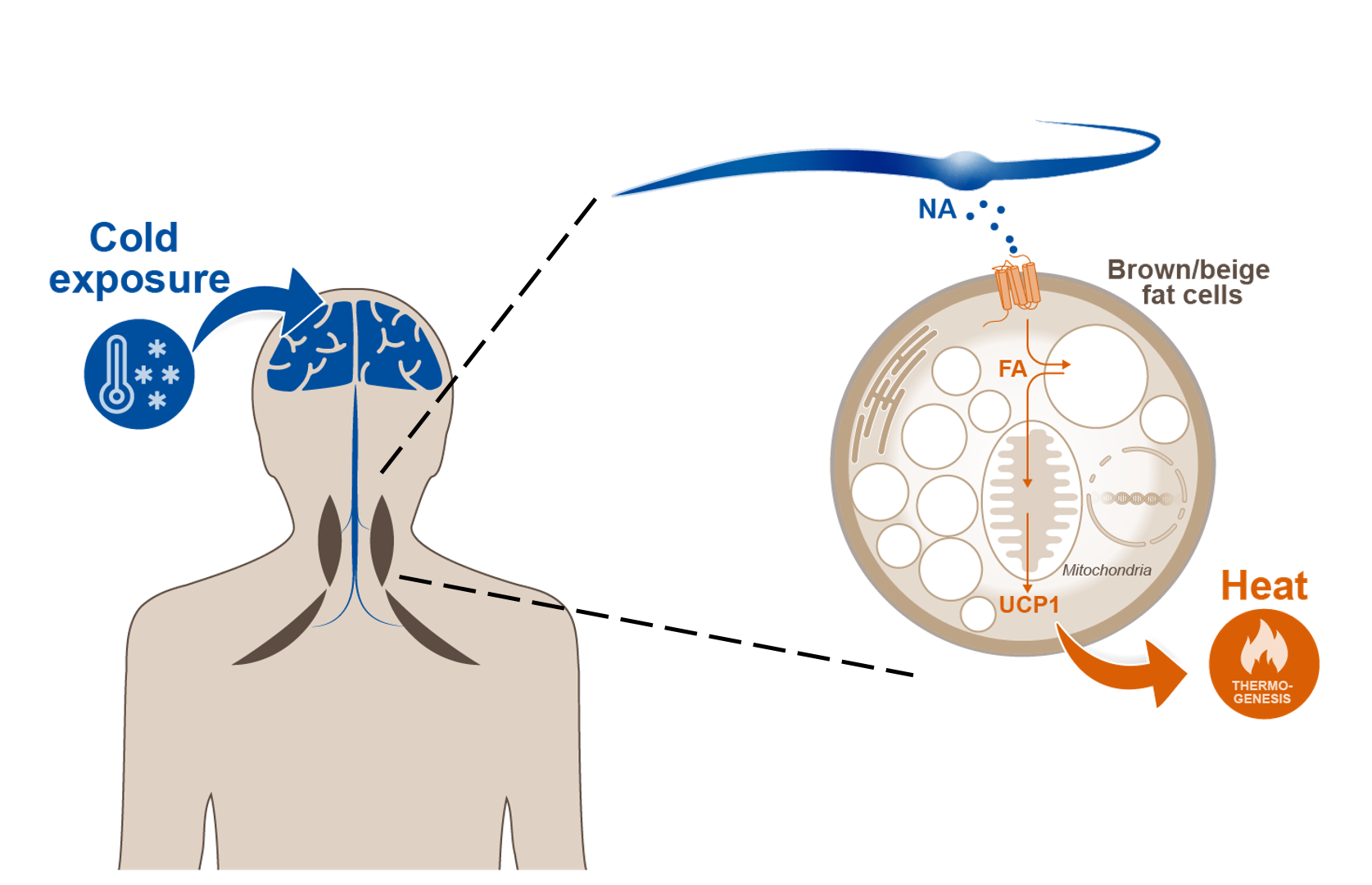
Adipose tissues play a central role in storage and release of energy. These tissues are organized in two major functionally distinct types, namely brown adipose tissue (BAT) and white adipose tissue (WAT). In contrast to white adipocytes, brown fat cells are specialized to dissipate energy in the form of heat. In addition to classical brown adipocytes in BAT, inducible brown adipocytes, referred to as brown-in-white (brite) or beige adipocytes, appear in WAT in response to various pharmacological stimuli or cold exposure. Together, these energy combusting brown and beige adipocytes constitute the thermogenic adipose tissue that is physiologically activated by cold exposure. Thermogenic fat has the potential to defend against obesity and its comorbidities by dissipating excess calories. Importantly, human BAT activity correlates with leanness and cardiometabolic health.
Investigating pathways of brown adipose tissue
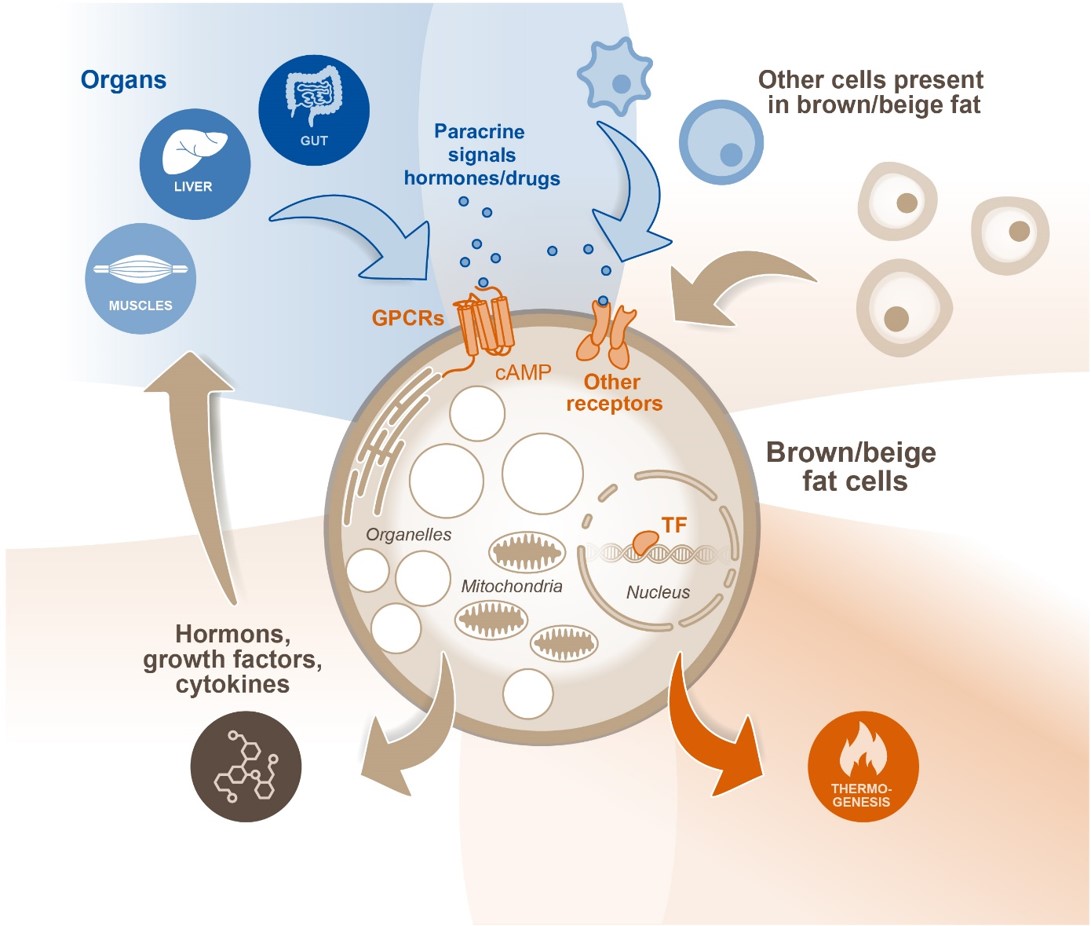
Understanding the regulation of brown/beige fat is essential for harnessing the metabolic power of thermogenic adipocytes. It is well known that BAT is activated by the sympathetic nervous system and adrenergic signaling. However, using this pathway is problematic because of cardiovascular side-effects. Recent studies, also by members of this consortium, have described alternative signals that control thermogenic fat. Another level of regulation is achieved by non-adipocytes, such as endothelial or immune cells, present in adipose tissues that secrete paracrine factors. The picture gets even more complex because brown/beige adipocytes themselves release factors that regulate energy dissipation in an autocrine manner. These, hormonal and metabolic signals are integrated in distinct signaling pathways and intracellular compartments. Thus, there is a new emerging picture of bidirectional communication und regulation between thermogenic adipocytes and other cell types as well as between thermogenic adipose tissue and other metabolic organs.
Combining expertise to provide excellent research
There is no single university or research center in Germany that can address these complex basic and clinically relevant questions on its own. For this reason, we established this consortium combining the internationally visible and complementary expertise of the three participating universities, the University of Bonn, Universität Hamburg and the Technical University of Munich. The Collaborative Research Centre/Transregio (CRC/TRR) brings together scientists from different fields, thus generating the synergism required to decipher the complex regulation of thermogenic energetics and its regulation. The research program focuses on
- the organ crosstalk between gut, liver, muscle and thermogenic adipose tissues,
- the cell-cell communication within brown and beige fat, and
- the interaction of cell organelles and intracellular signaling pathways in brown/beige adipocytes.
For this purpose, murine and human adipocytes and co-culture systems as well as novel transgenic mouse models will be utilized. Advanced technologies will be employed in the context of adaptive thermogenic responses, e.g. by measuring respiration and fuel oxidation from the subcellular to the systemic level. Molecular mechanistic studies will be performed under various environmental conditions such as housing temperature and high caloric diets, as well as in response to specific pharmacological stimuli to assess the relevance of thermogenic adipose tissues for metabolic adaptive responses and development of metabolic diseases.
Speakers of the TRR 333 and Steering Committee Members

Get to know the speakers and steering committee members of our TRR 333!
Coordination Office of the TRR 333

Get to know the members of our coordination teams of our TRR 333!
Find out more about our projects!
The TRR 333 consortium is a network of 19 projects, each TRR 333 project focuses on a certain aspect of metabolism in brown adipose tissue and beige adipose tissue.
Details of the CRC/TRR 333
General Information
Citys: Bonn; Hamburg; München
Funded: since 2022
Spokesperson
Professor Dr. Alexander Pfeifer
Vice Spokespersons
Professor Dr. Henriette Uhlenhaut
Professor Dr. Jörg Heeren
Applicant Institution
Rheinische Friedrich-Wilhelms-Universität Bonn (UB)
Co-Applicant Institution
Universität Hamburg (UHH)
Technische Universität München (TUM)
Participating Institution
Helmholtz Zentrum München (HHZ)
